“They succeed, because they think they can.” – Virgil, Poet (70 – 19 BC)
Appreciation
Tsk, tsk tsk… …What’s Up Doc?

A study published in the American Journal of Clinical Nutrition investigated how eating raw carrots affects blood lipids and colon function. In the study, adults consumed 200 g of raw carrots (about two medium–large carrots) every day at breakfast for three weeks. Researchers found that this dietary change reduced total serum cholesterol by about 11%. The results suggest that regularly eating raw carrots may help support heart health by lowering cholesterol levels in the blood.
The study also showed improvements in digestive function. Eating raw carrots increased fecal bile acid and fat excretion by about 50% and increased stool weight by around 25%. These changes suggest that carrots may help the body remove more bile acids and fats through stool, which can contribute to lower cholesterol levels. The researchers also noted that these effects persisted for about three weeks even after participants stopped eating the carrots, indicating a lasting impact on metabolism or gut bacteria.
PMID: 474479
Fasting-Body AND Brain
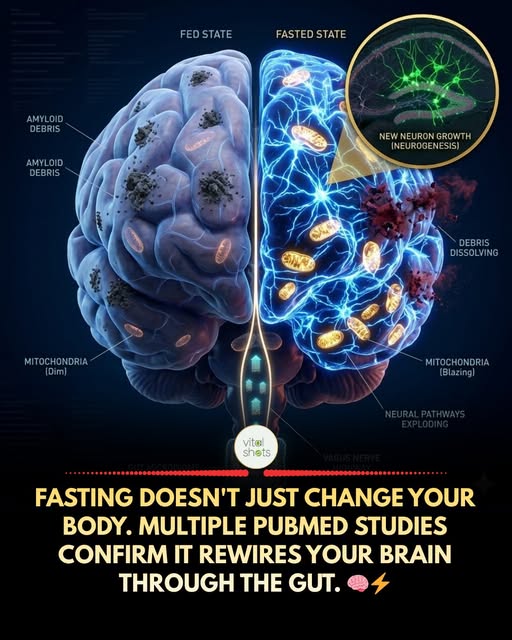
Most people try intermittent fasting because they want to lose weight. What they don’t realize is that some of the most compelling research on fasting has nothing to do with body fat.
It’s about what happens inside the skull.
A 2025 review published in PubMed (PMID:41356819) lays out the case in detail: intermittent fasting protects the brain through the gut-brain axis, not just through direct metabolic effects. When you fast, your gut microbiome composition shifts within days. Akkermansia muciniphila and other protective bacteria thrive during fasting windows. Short-chain fatty acids like butyrate rise. Intestinal barrier integrity improves. And from there, the brain feels it.
The mechanisms are specific. Fasting increases BDNF (brain-derived neurotrophic factor), often called “fertilizer for neurons,” which promotes hippocampal neurogenesis and synaptic plasticity. It reduces neuroinflammation via autophagy, a cellular “cleanup” process that clears damaged proteins including amyloid-beta, the same compound associated with Alzheimer’s progression. A separate 2025 paper (PMID:39798403) reviews evidence showing IF improves cognitive function in people with mild cognitive impairment, with the strongest mechanisms involving BDNF, CREB signaling, and reduced inflammatory cytokines in the brain.
One important nuance: duration matters significantly. Research from the gut-brain axis review indicates that short-term fasting protocols (around 8 weeks) are most effective at restoring gut barrier integrity and reducing systemic inflammation. Longer protocols (12+ weeks) begin to show more measurable effects on neurotrophic factors and actual cognitive performance. The two effects stack, which means consistency over time is more powerful than intensity in any single week.
The gut connection is what makes this genuinely new territory. For decades, fasting was studied almost entirely as a metabolic intervention. The idea that it works partly by growing better gut bacteria, which then signal the brain to repair and regenerate, reframes what we thought we understood about how lifestyle changes affect mental clarity, mood, and neurological resilience.
KEY FASTING PATTERNS STUDIED IN HUMANS
16:8 (16 hours fasting, 8 hours eating window) most common and sustainable protocol
5:2 (normal eating 5 days, very low calorie 2 days) studied specifically in older adults with insulin resistance (PMID:38901423)
Time-restricted eating aligned with morning light (earlier eating window) appears superior for circadian-based brain benefits
What does your current eating window look like? And have you ever noticed a difference in mental clarity or mood on days when you extended the fasting window?
Sources:
PMID:41356819 (IF and brain health via gut-brain axis, PubMed 2025).
PMID:39798403 (IF and neurocognitive disorders, PubMed 2025).
PMID:38901423 (IF in older adults with insulin resistance, PubMed 2024).
Vitamin B12 deficiency is probably the commonest cause of dementia and probably the easiest to cure
Vitamin B12 Deficiency is probably the commonest cause of dementia and probably the easiest to cure
It is reliably estimated that between 3% and 5% of the population are deficient in vitamin B12. Some experts put the figure as high as 10% and it is suggested that at least a fifth of all those over the age of 60 have low vitamin B12. The certainty is that vitamin B12 deficiency is an epidemic.
Moreover, it is an established fact that individuals who are deficient in vitamin B12 are likely to suffer from a wide range of symptoms with dementia being one of the most significant of those symptoms.
So, around the world, how many of the many millions said to be suffering from Alzheimer’s disease are in reality simply vitamin B12 deficient and could be cured with a short course of injections or a few vitamin tablets dissolved under the tongue?
We have to be talking about several hundred thousand patients in the UK alone. I’d suspect that the real figure is around 500,000.
If I am right that means that Alzheimer’s disease is nowhere near as common as it is said to be and that half a million patients with Alzheimer’s disease could have been cured with a simple two week course of injections.
Broken Mitochondria

Robert Lufkin MD reports on X:
I taught medical students that insulin resistance is a mystery. It’s not. A new study in Science Advances just identified the molecular trigger – mitochondrial oxidative stress.
Here’s what they found:
Lipid overload floods mitochondria with reactive oxygen species
This blocks GLUT4 – the glucose transporter your muscles need
Result: your cells can’t absorb sugar, even when insulin is screaming at them
Targeting mitochondrial oxidants improved insulin sensitivity by ~30%
This is the smoking gun for Type 2 diabetes.
We’ve spent decades blaming sugar and willpower. The real culprit is broken mitochondria – your cells’ power plants drowning in metabolic waste.
Fix the mitochondria, fix the insulin resistance. It’s that direct.
Full breakdown coming on the Health Longevity Secrets podcast.
Finish reading: https://www.science.org/doi/10.1126/sciadv.adq4461
(Tom: Here is my effort to provide the nutrition the body uses to repair mitochiondria: https://www.healthelicious.com.au/NutriBlast_DNA_Heart_Mitochondria.html)
Pick Your Weight

Your Family

Compared to many stories I hear I feel truly blessed.
Genetically Modified Microorganisms Can Collapse Ecosystems — But With Little or No Regulation, Anyone Can Create Them

(Tom: I received this from the Institute for Responsible Technology.)
Dear IRT Community,
We are pleased to share that Children’s Health Defense has published an excellent review of Jeffrey Smith’s latest peer-reviewed research report on genetically modified microorganisms and the concerning regulatory gaps surrounding their development.
This report, which Jeffrey co-authored with other leading experts in the field, examines how these microscopic organisms are being created and released with minimal safety testing or regulatory oversight. Our research documents the potential for these genetically engineered microbes to spread through environmental systems in ways that are difficult to predict or control.
The Children’s Health Defense article does an excellent job summarizing our key findings, including the regulatory gaps that echo the same patterns we’ve documented throughout the biotech industry – insufficient testing, rushed approvals, and inadequate long-term safety studies.
Our peer-reviewed research raises important questions that need broader public discussion. We encourage you to read this accessible summary of our work and share it with others who care about responsible biotechnology.
Immunize Yourself Against Control Techniques

